The white solid begins to disappear into the solution but it does not dissolve completely. Chemistry questions and answers.

Ziplock Chemistry Thermodynamics
Place 1 level spoonful of Calcium chloride in the other corner of the bag.

. Immediately work the air out of the bag and seal it. The contents if the vial MUST NOT SPILL. One chemical used in a plastic bag is called a petroleum.
Open the bag while a VSVS member goes around and adds a teaspoon of calcium chloride. Pour 10 mL of cabbage juice into a. Put 3 teaspoons of baking soda into the middle of the paper towel and fold the paper towel in halves to form a.
Gently shake the contents of the bag while holding the bag over the plate. Do not allow them to touch. Calcium chloride and phenol red are mixed together in a Ziploc bag.
Lay the plastic bag on the lab counter. Goggles must be worn at all times in the laboratory. Hold a reaction in your hand literally as students observe and ask questionsThis video is part of the Flinn Scientific Best Practices for Teaching Chemist.
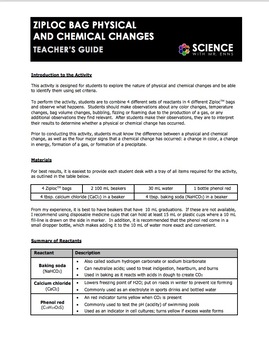
Chemistry in a Ziploc Bag In this experiment students will have the opportunity to observe some chemicals and the chemical reactions that occur when these chemicals are combined in a closed container. 35mm film canister top optional Small paper cup. The final mixture is slightly cloudy.
Give each pair one ziploc bag containing baking soda one 1 oz cup containing 15 mL of phenol red solution and one plate. State your answer to the question as a hypothesis remember I think is wimpy _____ _____ Materials. 30 mL Indicator solution phenol red 30 mL red cabbage juice Procedure 1.
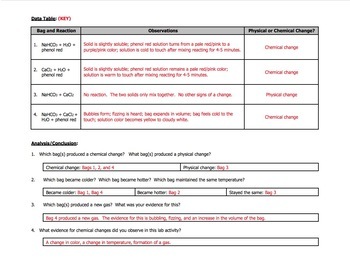
The bag begins to be quite hot. Calcium Chloride mostly dissolves. In addition to an exothermic reaction occurring in the Ziploc bag an endothermic reaction took place as well once the chemicals returned to their starting temperature.
4 Ziploc bags. 1-gallon bromothymol blue indicator 10-ml graduated cylinders one per lab group teaspoons 1 to 2 per lab group 3 pounds calcium chloride CaCl 2 from chemical supply house or from a store selling this type of road salt or laundry aid 1-12 pounds sodium bicarbonate NaHCO 3 baking soda Activities. Open the bag while a VSVS member goes around and adds a teaspoon of calcium chloride.
When three substances are mixed in a sealed clear plastic bag chemical reactions occur that causes. Up to 24 cash back Put one scoop full of baking soda in a zip lock bag Put two scoops of calcium chloride in the same bag. Place 1 level spoonful of Sodium bicarbonate in one corner of the bag.
Up to 24 cash back. Measure 10 ml-a of bromothymol blue into a plastic vial. For common commercial grades of medium- and high-density polyethylene Ziploc the melting point is typically in the range 120 to 130 C 250 to 265 F.
Hold the bag upright over the plate. You can do this experiment outside or in a sink 2. Add 2 drops of the 02 phenol red solution to the water.
Place 1 spoon of NaHCO3 baking soda in the left hand corner of the bag and 1 spoon of CaCl2 calcium chloride in the right corner of the bag. This 5-page Ziploc Bag Physical and Chemical Changes lab activity is designed to give students experience observing different chemical reactions and identify them as physical or chemical changes according to set criteriaTo perform the activity students combine different substances baking soda c. Though the temperature change of the endothermic reaction was nowhere was near how much the exothermic reaction changed the Ziploc bags temperature it was significant enough to be noticed from touching.
30 mL water. Calcium chloride is hygroscopic and will pick up wate r from the air. Heres a brief introduction to how chemists make modern plastics possible.
Lab - Ziploc Bag Physical and Chemical Changes. Measure one spoonful of calcium chloride and place it into the ziplock bag. Calcium chloride anhydrous CaCl2.
Phenol red solution 2 plastic teaspoons 2 10-mL containers vials 6 Zip-Loc bags quart size. Zip the bag closed and shake it to observe for any evidence of a chemical change. Petroleum is then usually mixed in with other natural gases to makea plastic bag.
Plastics are the result of the very real marriage of raw materials engineering and energyall brought together through chemistry. Add the phenol red solution including water to the bag and seal the bag. The bag feels cold because baking soda absorbs heat when it dissolves in water.
If any bromothymol blue is dripping down the outside of the vial use paper towel to wipe this off VERY CAREFULLY place the vial UPRIGHT in the bag. No worries if you didnt ace your chemistry class. Add the phenol red solution including water to the bag and seal the bag.
1 quart Ziploc bag 1 gallon Ziploc bag 12 cup milk 12 cup whipping cream 14 cup sugar 14 teaspoon vanilla flavoring sodium chloride rock salt ice thermometer measuring cups 1 12 and 14 cups Styrofoam cups plastic spoons Procedure 1. Baking soda sodium bicarbonate NaHCO3. The final color is red no change from the initial color.
While plastics can be high tech advanced materials. Aluminum Pan Ziploc bag of calcium chloride Baking soda Phenol red solution 10 ml graduated cylinder Safety goggles Mini-spoon Thermometer Procedure. Hold the bag upright over the plate.
Always wear eye protection and gloves when doing chemistry experiments. In the final observation Baking Soda and Phenol Red was mixed. Add one spoonful of sodium bicarbonate baking soda to the bag.
Sodium hydrogencarbonate to a Ziploc bag. Put ½ cup of vinegar in Ziploc bag. When the anhydrous calcium chloride the mixture becomes warm at first because anhydrous calcium chloride gives off heat when it dissolves in water.
About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy Safety How YouTube works Test new features Press Copyright Contact us Creators. Into a one-quart Ziploc bag place 14 cup sugar 12 cup milk 12 cup. Sodium hydrogencarbonate Baking soda.
The phenol red solution then turns yellow which indicates an acidic solution.
Lab Ziploc Bag Physical And Chemical Changes By Science With Mr Enns

Title Chemistry In A Ziploc Bag Pages 1 5 Flip Pdf Download Fliphtml5

Title Chemistry In A Ziploc Bag Pages 1 5 Flip Pdf Download Fliphtml5

Lab Ziploc Bag Physical And Chemical Changes Chemical Changes Chemical And Physical Changes Chemical Changes Lab

Law Of Conservation Of Mass Matter Exit Ticket Conservation Of Mass Exit Tickets Middle School Science Resources
Lab Ziploc Bag Physical And Chemical Changes By Science With Mr Enns

0 comments
Post a Comment